
Chemistry, 09.03.2021 22:00 lilblackbird4
In the unbalanced equation shown below, how much iron was present before the reaction if there was 20 g of oxygen and 67 g of iron oxide produced?
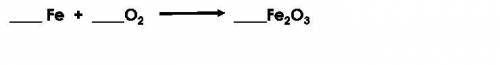

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, vapelordcarl69
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 16:00, annsmith66
What statement goes against the kinetic theory of gases
Answers: 1


Chemistry, 23.06.2019 01:00, Alysssssssssssa
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1
You know the right answer?
In the unbalanced equation shown below, how much iron was present before the reaction if there was 2...
Questions in other subjects:


History, 13.05.2021 23:40

Mathematics, 13.05.2021 23:40


Mathematics, 13.05.2021 23:40



Mathematics, 13.05.2021 23:40


World Languages, 13.05.2021 23:40



