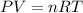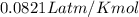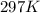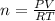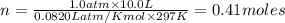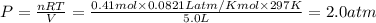Chemistry, 09.03.2021 20:10 2020davidhines
A sample of nitrogen (N2) gas in a 10.0L container has a
pressure of 1.0 atm at 297 K. Assuming ideal gas behavior,
what will the pressure be if the same amount of nitrogen
gas is put into a 5.0L container at 297 K?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 22.06.2019 22:00, robert7248
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 23.06.2019 02:00, ItzAquaZ1449
Alice did an experiment to find the relationship between the angle at which a ray of light strikes a mirror and the angle at which the mirror reflects the light. she placed a ray box in front of a mirror. she changed the angle at which the light from the ray box struck the mirror and noted the corresponding angle at which the mirror reflected the light. which of the following is the dependent variable in this experiment? the mirror used to reflect the light the ray box used as the source of light angle at which the light from the ray box strikes the mirror angle at which the mirror reflects the light from the ray box
Answers: 2
You know the right answer?
A sample of nitrogen (N2) gas in a 10.0L container has a
pressure of 1.0 atm at 297 K. Assuming ide...
Questions in other subjects:

Mathematics, 13.07.2020 20:01

English, 13.07.2020 20:01

Mathematics, 13.07.2020 20:01

Mathematics, 13.07.2020 20:01

Mathematics, 13.07.2020 20:01

World Languages, 13.07.2020 20:01


Social Studies, 13.07.2020 20:01


Mathematics, 13.07.2020 20:01