Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, smm1106
Alculate the concentration of h3o⁺in a solution that contains 5.5 × 10-5m oh⁻at 25°c. identify the solution as acidic, basic, or neutral. a) 1.8 × 10-10m, basicb) 1.8 × 10-10m, acidicc) 5.5 × 10-10m, neutrald) 9.2 × 10-1m, acidice) 9.2 × 10-1m, basic
Answers: 1

Chemistry, 21.06.2019 18:00, mykalwashington
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 21.06.2019 23:50, scavalieri2421
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 02:30, brittanysanders
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2
You know the right answer?
0.2:
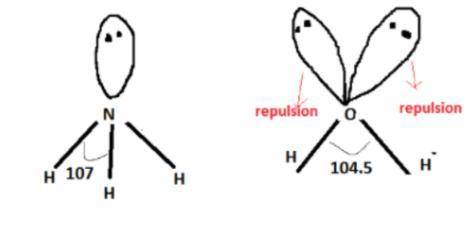
The bond angles of H20 and NH3 are not 109.5" like that of CH4, although O and N
atoms ar...
atoms ar...
Questions in other subjects:


Mathematics, 30.07.2019 11:00

Mathematics, 30.07.2019 11:00

History, 30.07.2019 11:00

Mathematics, 30.07.2019 11:00


Mathematics, 30.07.2019 11:00


Geography, 30.07.2019 11:00

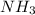 , nitrogen is the central atom and there are 3 Hydrogens as monovalent atoms. The number of electron pairs is 4 that means the hybridization will be
, nitrogen is the central atom and there are 3 Hydrogens as monovalent atoms. The number of electron pairs is 4 that means the hybridization will be 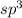 and geometry of the molecule will be pyramidal as there are three bond pairs and one lone pair.The bond angle for pyramidal geometry is 107°. The bond angle reduces due to lone pair bond pair repulsions.
and geometry of the molecule will be pyramidal as there are three bond pairs and one lone pair.The bond angle for pyramidal geometry is 107°. The bond angle reduces due to lone pair bond pair repulsions.
 , oxygen is the central atom and there are 2 Hydrogens as monovalent atoms.
The number of electron pairs is 4 that means the hybridization will be
, oxygen is the central atom and there are 2 Hydrogens as monovalent atoms.
The number of electron pairs is 4 that means the hybridization will be