
Chemistry, 08.03.2021 14:00 24hudsonmoss
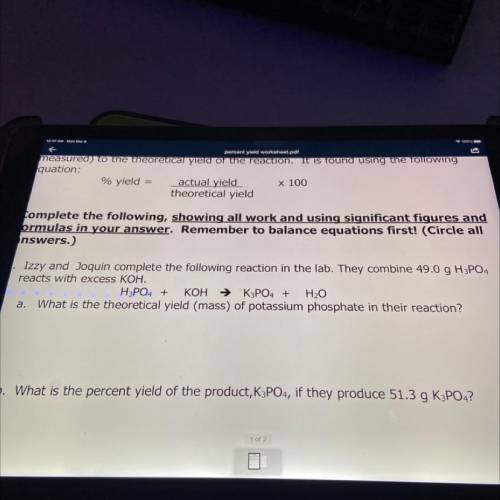
Izzy and Joquin complete the following reaction in the lab. They combine 49.0 g H3PO4
reacts with excess KOH.
H3PO4 + KOH → K3PO4 + H2O
a. What is the theoretical yield (mass) of potassium phosphate in their reaction?
b. What is the percent yield of the product, K3PO4, if they produce 51.3 g K3PO4?
1 of 2

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 22.06.2019 23:30, bxymichelle
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3

Chemistry, 23.06.2019 14:00, jasminebaeeecx
If the molar mass of the compound is 96.69 g/mol, what is the molecular formula of the compound?
Answers: 1
You know the right answer?
Izzy and Joquin complete the following reaction in the lab. They combine 49.0 g H3PO4
reacts with e...
Questions in other subjects:


Mathematics, 06.07.2019 12:30

Mathematics, 06.07.2019 12:30



Chemistry, 06.07.2019 12:30

Social Studies, 06.07.2019 12:30

Chemistry, 06.07.2019 12:30

Mathematics, 06.07.2019 12:30

Mathematics, 06.07.2019 12:30



