
Please help , don’t answer if u don’t know please
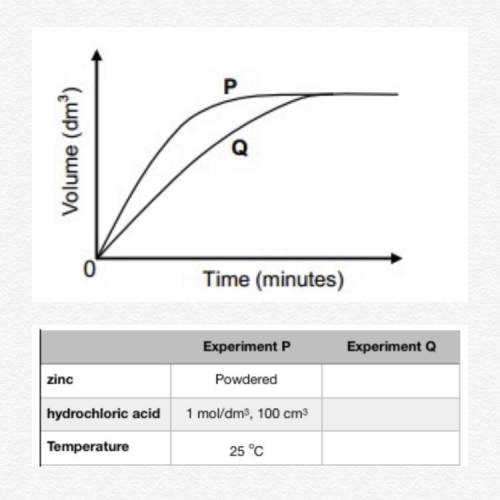
An experiment is carried out to investigate the rate at which hydrogen gas is produced when a given amount of zinc reacts with an excess of a dilute hydrochloric acid solution. Curve P on the graph below shows the volume of hydrogen gas formed when 100 cm3 of a 1 mol/dm3 solution is used. (Reaction rate is indicated by the slope of the graphs.)
Zn + 2 HCl = ZnCl2 + H2
a. Design an experiment that would produce curve Q on the graph below. (Hint: change the experimental parameters so that the rate of gas evolution changes according to the curves). You can also use the table to record your data.
b. What is the implication of the fact that the two curves touch? Explain.
c. Give a reason why the rate of hydrogen production slows down towards the end in both experiments.

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:30, shookiegriffin
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 22.06.2019 22:30, pookie879
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
Please help , don’t answer if u don’t know please
An experiment is carried out to investigate the r...
Questions in other subjects:



Mathematics, 07.10.2019 20:20




Mathematics, 07.10.2019 20:20





