Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:00, breannaasmith1122
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 03:10, emilyplays474
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2
You know the right answer?
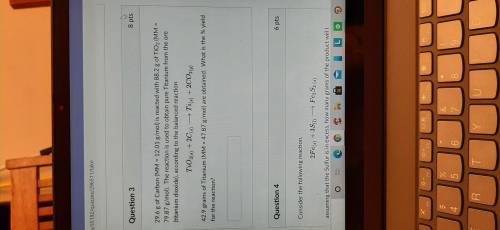
29.6 g of Carbon (MM = 12.01 g/mol) is reacted with 88.2 g of TiO2 (MM= 79.87 g/mol). 42.9 grams of...
Questions in other subjects:

History, 11.07.2019 17:00

Biology, 11.07.2019 17:00


Computers and Technology, 11.07.2019 17:00



Mathematics, 11.07.2019 17:00