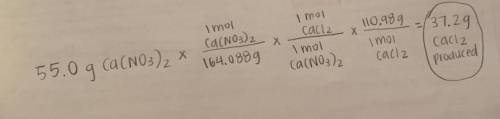Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, badgirl2005
This is a mixture that has the same composition throughout.
Answers: 1



Chemistry, 23.06.2019 10:30, krlx
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle. if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3.2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
Ca(NO3)2 + HCl --> HNO3 + CaCl2
According to the reaction above, 55.0 grams of calcium nitrate r...
Questions in other subjects:

Social Studies, 31.07.2019 18:00


Mathematics, 31.07.2019 18:00


Social Studies, 31.07.2019 18:00

Mathematics, 31.07.2019 18:00


Mathematics, 31.07.2019 18:00

Mathematics, 31.07.2019 18:00

History, 31.07.2019 18:00