Determine the molecular formula for the compounds below.
C2H4O ; molar mass=176 g/mol
...

Chemistry, 06.03.2021 03:20 mariahcid904
Determine the molecular formula for the compounds below.
C2H4O ; molar mass=176 g/mol
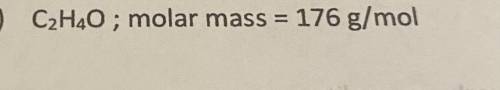

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, froyg1234
Read the given expression. x = number of protons − number of core electrons which of the following explains the identity of x and its trends across a period? x is the effective nuclear charge, and it remains constant across a period. x is the screening constant, and it remains constant across a period. x is the effective nuclear charge, and it increases across a period. x is the screening constant, and it increases across a period.
Answers: 1

Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1


Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1
You know the right answer?
Questions in other subjects:

Biology, 02.08.2019 19:30

Biology, 02.08.2019 19:30

Physics, 02.08.2019 19:30

History, 02.08.2019 19:30

History, 02.08.2019 19:30

Chemistry, 02.08.2019 19:30

History, 02.08.2019 19:30

Mathematics, 02.08.2019 19:30

Mathematics, 02.08.2019 19:30

Mathematics, 02.08.2019 19:30



