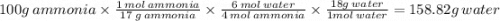Given the reaction:
4 NH3 + 5 O2 → 4 NO + 6 H2O
If you start with 100.0 grams of NH3, how man...


Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, TamB01
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2


Chemistry, 22.06.2019 11:40, arlabbe0606
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3
You know the right answer?
Questions in other subjects:

Computers and Technology, 21.01.2021 22:50

Mathematics, 21.01.2021 22:50


Physics, 21.01.2021 22:50





Mathematics, 21.01.2021 22:50