Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 06:40, alyons60
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 15:30, elizabethprasad2
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1

You know the right answer?
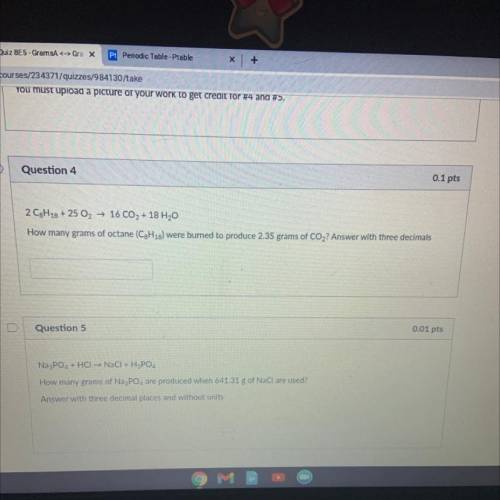
2 C8H18 + 25 O2 → 16 CO2 + 18 H20
How many grams of octane (C3H18) were burned to produce 2.35 gram...
Questions in other subjects:



History, 12.05.2021 01:00


Mathematics, 12.05.2021 01:00

Mathematics, 12.05.2021 01:00


History, 12.05.2021 01:00

Advanced Placement (AP), 12.05.2021 01:00