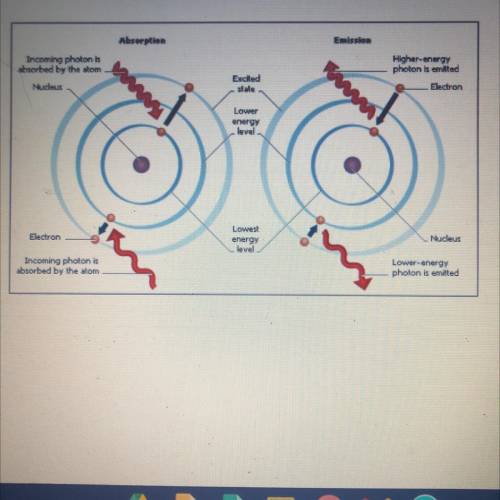
Q.1 When a metal is heated, what
happens to the electrons in
the atoms?
Q.2 What is th...


Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:30, hdhtvthjr
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 13:50, hannahmyung1113
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3
You know the right answer?
Questions in other subjects:


Mathematics, 05.05.2020 05:20

Mathematics, 05.05.2020 05:20




Mathematics, 05.05.2020 05:20

Geography, 05.05.2020 05:20

Mathematics, 05.05.2020 05:20