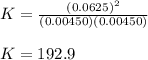Chemistry, 05.03.2021 14:00 hoopstarw4438
At equilibrium at 2500K, [HCl]=0.0625M and [H2]=[Cl2]=0.00450M for the reaction H2+Cl2 ⇌ HCl.
a. Determine the balanced equation and write the equilibrium expression
b. Determine the K eq
c. Will this process favor the reactants or products at equilibrium?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3


Chemistry, 23.06.2019 02:00, raulflores01
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1
You know the right answer?
At equilibrium at 2500K, [HCl]=0.0625M and [H2]=[Cl2]=0.00450M for the reaction H2+Cl2 ⇌ HCl.
a. De...
Questions in other subjects:

Chemistry, 11.09.2019 02:20

Mathematics, 11.09.2019 02:20



Mathematics, 11.09.2019 02:20

Social Studies, 11.09.2019 02:20


Mathematics, 11.09.2019 02:20


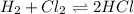
![K=\frac{[HCl]^2}{[H_2][Cl_2]}](/tpl/images/1171/4277/48ea9.png)