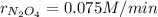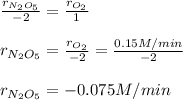Chemistry, 05.03.2021 14:00 knussoshd3603
Given the following reaction: 2N2O5=2N2O4+O2, if the rate of oxygen production is 0.15M/min, determine:
a. The rate that N2O5 is consumed
b. The rate that N2O4 is produced

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, sairaanwar67
Which substance absorbs 58.16 kj of energy when 3.11 mol vaporizes? a)ch4 b)h2s c)co2 d)nacl
Answers: 2

Chemistry, 22.06.2019 05:00, Ashleyvasquez2261
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 13:30, xojade
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 19:00, Farhan54019
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1
You know the right answer?
Given the following reaction: 2N2O5=2N2O4+O2, if the rate of oxygen production is 0.15M/min, determi...
Questions in other subjects:


History, 05.05.2020 01:06

Mathematics, 05.05.2020 01:06

Mathematics, 05.05.2020 01:06





Mathematics, 05.05.2020 01:06