
Chemistry, 05.03.2021 06:50 rleiphart1
When 1.50 mol CO2 and 1.50 mol H2 are placed in a 3.00-Lcontainer at 395 °C, the following reaction
CO2(g) + H2(g) = CO(g) + H2O(g). If Kc = 0.802,
what are the concentrations of each substance in the equilibrium mixture?
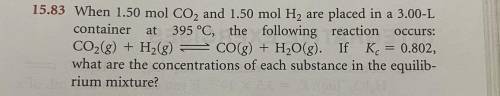

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, bryneosburn
What’s a special glass that most beakers are made of
Answers: 1

Chemistry, 22.06.2019 11:40, tatemelliott
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 21:00, Janznznz4012
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
When 1.50 mol CO2 and 1.50 mol H2 are placed in a 3.00-Lcontainer at 395 °C, the following reaction...
Questions in other subjects:

History, 14.08.2020 21:01





English, 14.08.2020 21:01

English, 14.08.2020 21:01

Mathematics, 14.08.2020 21:01

Mathematics, 14.08.2020 21:01

English, 14.08.2020 21:01



