
Chemistry, 05.03.2021 01:40 xandraeden32
Which Statement describes my sodium react more by vigorously then magnesium to hydrochloride acid
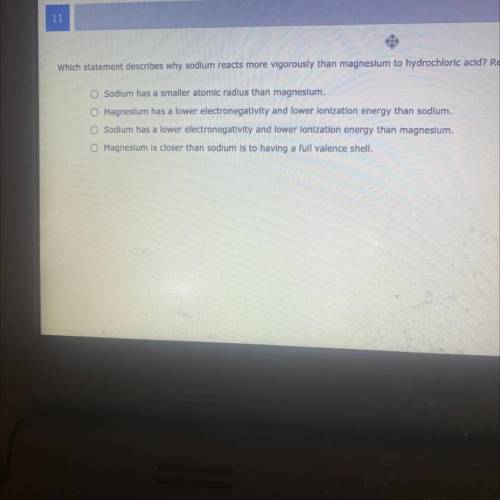

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, kevinhill185
Pauling and lewis questioned the extreme definitions of bonds. they wondered if bonds might be described somewhere in between the two extremes (covalent and ionic). on the basis of experimental data, pauling confirmed that bonds could be ionic, covalent, and for those, in between, exhibit a degree of ionic character. he theorized that the major factor was how strongly the atoms in the bond attracted the electrons. pauling called this factor - the tendency of an atom to attract electrons in a bond.
Answers: 2


Chemistry, 22.06.2019 18:10, ellemarshall13
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 22.06.2019 20:00, emilyswinge4421
Listenbase your answer to the question on the information below. nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body. cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment. which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2
You know the right answer?
Which Statement describes my sodium react more by vigorously then magnesium to hydrochloride acid
Questions in other subjects:



History, 31.03.2021 04:40

Mathematics, 31.03.2021 04:40

Chemistry, 31.03.2021 04:40



Mathematics, 31.03.2021 04:40


Mathematics, 31.03.2021 04:40



