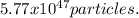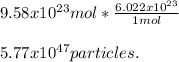Chemistry, 05.03.2021 01:10 winterblanco
How many particles are in 9.58 x 1023 of potassium sulfate (K2SO4)? (5.77 x 1047 particles)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, ciarakelly636owuiup
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1

Chemistry, 22.06.2019 03:00, actheorian8142
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 06:30, Pizzapegasus1
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 09:20, pandaman632
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
How many particles are in 9.58 x 1023 of potassium sulfate (K2SO4)? (5.77 x 1047 particles)...
Questions in other subjects:


Mathematics, 08.12.2020 18:50

Mathematics, 08.12.2020 18:50


Chemistry, 08.12.2020 18:50


English, 08.12.2020 18:50

Mathematics, 08.12.2020 18:50


Mathematics, 08.12.2020 18:50