
Chemistry, 04.03.2021 23:40 kingjames82
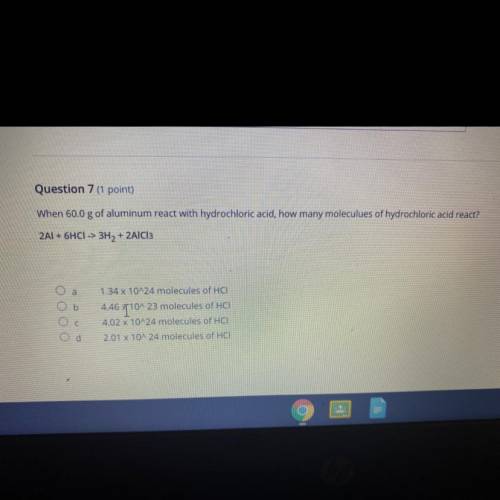
Question 7 (1 point)
When 60.0 g of aluminum react with hydrochloric acid, how many moleculues of hydrochloric acid react?
2Al + 6HCI -> 3H2 + 2AlCl3
a
b
ОООО
1.34 x 10^24 molecules of HCI
4.46 T10^23 molecules of HCI
4.02 x 10^24 molecules of HCI
2.01 X 10^24 molecules of HCI

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, 20alondra04
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1


Chemistry, 23.06.2019 01:00, Zachgrainger4436
You wish to prepare a buffer consisting of acetic acid and sodium acetate with a total acetic acetate plus acetate concentration of 250 mm and a ph of 5. what concentrations of acetic acid and sodium acetate should you use
Answers: 1

Chemistry, 23.06.2019 04:31, CassidgTab
Which molecules are more strongly attracted to one another -c3h8o molecules that make up liquid rubbing alcohol or ch4 molecules that make up methane gas
Answers: 3
You know the right answer?
Question 7 (1 point)
When 60.0 g of aluminum react with hydrochloric acid, how many moleculues of h...
Questions in other subjects:




Biology, 23.04.2020 19:14


World Languages, 23.04.2020 19:14


Mathematics, 23.04.2020 19:14


English, 23.04.2020 19:14



