
Chemistry, 04.03.2021 23:20 chicalapingpon1938
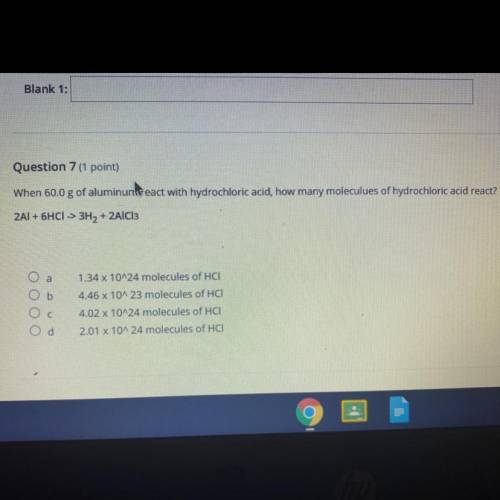
Question 7 (1 point)
When 60.0 g of aluminum react with hydrochloric acid, how many moleculues of hydrochloric acid react?
2AI + 6HCI -> 3H2 + 2AlCl3
O a
1.34 x 10^24 molecules of HCI-
Ob
Ос
4.46 x 10A 23 molecules of HCI
4.02 x 10^24 molecules of HCI:
2.01 x 10^24 molecules of HCI

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, esnyderquintero
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 14:30, Hannahmiller3773
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 22.06.2019 20:00, Isaiahtate053
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3
You know the right answer?
Question 7 (1 point)
When 60.0 g of aluminum react with hydrochloric acid, how many moleculues of h...
Questions in other subjects:

History, 15.01.2021 20:10

Business, 15.01.2021 20:10

Mathematics, 15.01.2021 20:10

English, 15.01.2021 20:10


Mathematics, 15.01.2021 20:10

Mathematics, 15.01.2021 20:10





