
Chemistry, 04.03.2021 21:40 kerstynsharp08
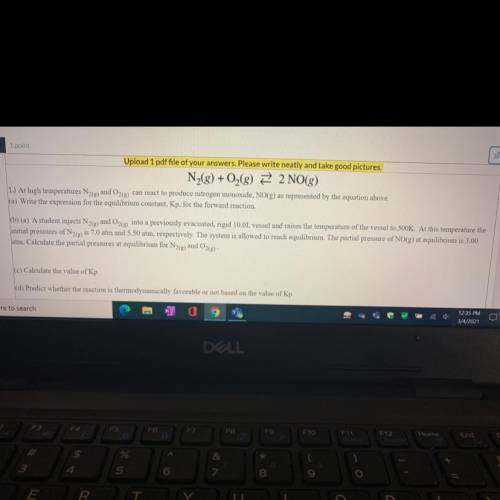
N2 + O2 —> 2NO
b) A student injects N2(g)and O2(g) into a previously evacuated, rigid 10.0L vessel and raises the temperature of the vessel to 500K. At this temperature the
initial pressures of N2(g) is 7.0 atm and 5.50 atm, respectively. The system is allowed to reach equilibrium. The partial pressure of NO(g) at equilibrium is 3.00
atm. Calculate the partial pressures at equilibrium for N2(e) and O2(g)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, Bryanguzman2004
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 15:20, munziruddin204
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 17:50, mytymikey123
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2

Chemistry, 23.06.2019 00:00, samangelzrose3576
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1
You know the right answer?
N2 + O2 —> 2NO
b) A student injects N2(g)and O2(g) into a previously evacuated, rigid 10.0L vess...
Questions in other subjects:

Mathematics, 21.07.2019 02:00




History, 21.07.2019 02:00


Chemistry, 21.07.2019 02:00






