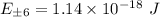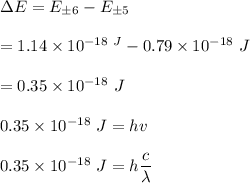The particle on a ring is a useful model for the motion of electrons around the porphine ring, the conjugated macrocycle that forms the structural basis of the heme group and the chlorophylls. We may treat the group as a circular ring of radius 440 pm, with 22 electrons in the conjugation system moving along the perimeter of the ring. In the ground state of the molecules each state is occupied by two electrons.
A) Calculate the energy and angular momentum of an electron in the highest occupied level.
B) Calculate the frequency of radiation that can induce a transition between the highest occupied and lowest unoccupied levels.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:00, officialgraciela67
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 10:30, Riplilpeep
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

You know the right answer?
The particle on a ring is a useful model for the motion of electrons around the porphine ring, the c...
Questions in other subjects:

Mathematics, 08.01.2021 05:10



Mathematics, 08.01.2021 05:10

Mathematics, 08.01.2021 05:10

Mathematics, 08.01.2021 05:10

Mathematics, 08.01.2021 05:10



Mathematics, 08.01.2021 05:10

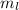 can be computed by using the formula:
can be computed by using the formula:

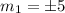

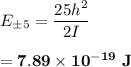

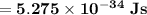
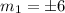 which implies that the energy
which implies that the energy