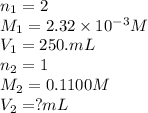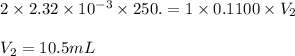Achemistry student weighs out 0.0475g of sulfurous acid h2so3, a diprotic acid, into a
250.ml...

Achemistry student weighs out 0.0475g of sulfurous acid h2so3, a diprotic acid, into a
250.ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1100m naoh solution.
calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, reaperqueen21
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 20:00, montimcdaniel
If one fission reaction of a uranium-235 atom produced two neutrons, how many neutrons would be released if the chain reaction occurred three more times?
Answers: 1

Chemistry, 22.06.2019 22:30, kiera2599
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1

Chemistry, 23.06.2019 00:00, jasmin5285
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 21.10.2020 22:01


History, 21.10.2020 22:01


History, 21.10.2020 22:01

English, 21.10.2020 22:01

Biology, 21.10.2020 22:01


Chemistry, 21.10.2020 22:01


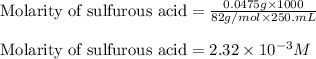
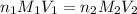
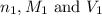 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 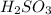
 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.