Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:10, akatsionis25
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3

Chemistry, 22.06.2019 08:30, MacenParisi
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1
You know the right answer?
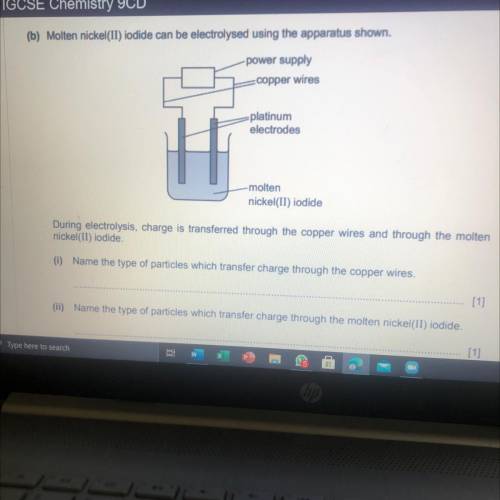
Predict the products of the electrolysis of molten nickel(II) iodide. Write an ionic half-equation f...
Questions in other subjects:

English, 12.03.2021 19:40

Mathematics, 12.03.2021 19:40

Mathematics, 12.03.2021 19:40

Mathematics, 12.03.2021 19:40




History, 12.03.2021 19:40