
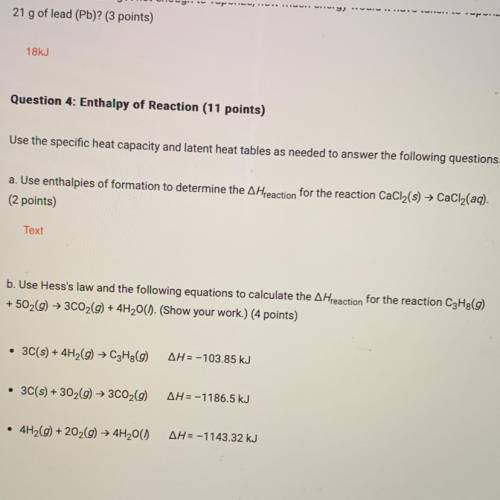
B. Use Hess's law and the following equations to calculate the A Hreaction for the reaction C3H8(9)
+ 502(9) + 3C02(9) + 4H2O(). (Show your work.) (4 points)
• 3C(s) + 4H2(9) → C3Hg(9)
AH = -103.85 kJ
• 3C(s) + 302(g) + 3C02(9)
AH=-1186.5 kJ
• 4H2(9) +202(9) + 4H2O()
AH=-1143.32 kJ

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, thechocolatblanc
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2

Chemistry, 22.06.2019 18:30, losalobos46
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
B. Use Hess's law and the following equations to calculate the A Hreaction for the reaction C3H8(9)...
Questions in other subjects:



Biology, 12.04.2021 19:00

Social Studies, 12.04.2021 19:00








