Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 23.06.2019 01:30, Thunderalesis7855
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1
You know the right answer?
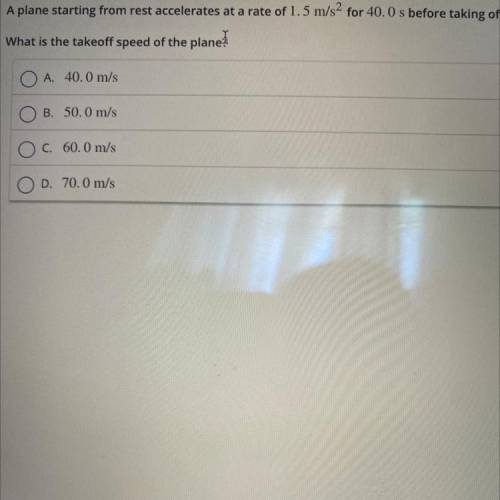
A plane starting from rest accelerates at a rate of 1.5 m/s2 for 40.0 s before taking off.
What is...
Questions in other subjects:



Mathematics, 07.08.2021 18:30


Social Studies, 07.08.2021 18:30

Engineering, 07.08.2021 18:30


Mathematics, 07.08.2021 18:30


English, 07.08.2021 18:30