
Chemistry, 03.03.2021 18:50 alexj29227405
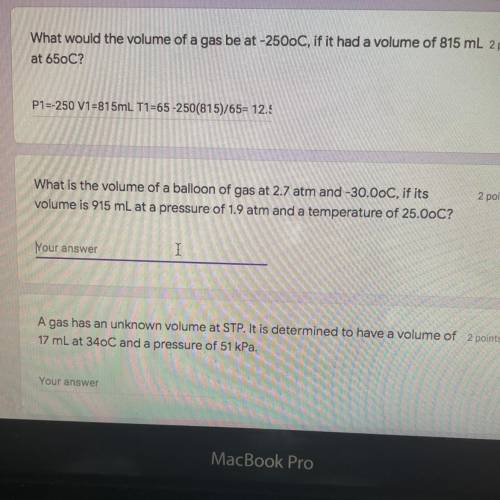
What is the volume of a balloon of gas at 2.7 atm and -30.0oC if it’s volume is 915mL at a pressure of 1.9atm and a temperature of 25.0oC?

Answers: 2


Other questions on the subject: Chemistry




Chemistry, 23.06.2019 04:31, mdarter
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2
You know the right answer?
What is the volume of a balloon of gas at 2.7 atm and -30.0oC if it’s volume is 915mL at a pressure...
Questions in other subjects:

Mathematics, 25.07.2019 12:00


Mathematics, 25.07.2019 12:00

Mathematics, 25.07.2019 12:00


Biology, 25.07.2019 12:00






