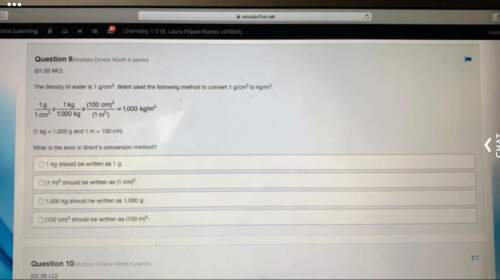
The following reaction shows sodium hydroxide reacting with sulfuric acid.
4NaOH + 2H2SO4 → 2Na2SO4 + 4H2O
How many grams of Na2SO4 are produced from 10.0 grams of NaOH?
(Molar mass of Na = 22.989 g/mol, O = 15.999 g/mol, H = 1.008 g/mol, S = 32.065 g/mol) (5 points)
17.8 grams
19.2 grams
35.5 grams
38.5 grams

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:00, AbhiramAkella
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

You know the right answer?
The following reaction shows sodium hydroxide reacting with sulfuric acid.
4NaOH + 2H2SO4 → 2Na2SO4...
Questions in other subjects:


Mathematics, 19.10.2020 01:01

Mathematics, 19.10.2020 01:01




Biology, 19.10.2020 01:01


Biology, 19.10.2020 01:01

English, 19.10.2020 01:01