
Chemistry, 20.09.2019 19:00 arthurdolz
For the reaction shown, calculate how many grams of oxygen form when each quantity of reactant completely reacts.
2 kclo3(s) → 2 kcl(s) + 3 o2(g)
a. 2.72 g kclo3
b. 0.361 g kclo3
c. 83.6 kg kclo3
d. 22.5 mg kclo3

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, badgirl2005
This is a mixture that has the same composition throughout.
Answers: 1


Chemistry, 22.06.2019 14:00, JJlover1892
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
For the reaction shown, calculate how many grams of oxygen form when each quantity of reactant compl...
Questions in other subjects:

Mathematics, 09.03.2021 04:50

Biology, 09.03.2021 04:50

Mathematics, 09.03.2021 04:50

Mathematics, 09.03.2021 04:50




Mathematics, 09.03.2021 04:50


 = 39.1 + 35.5 + 3(16.0) = 122.6 g
= 39.1 + 35.5 + 3(16.0) = 122.6 g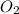 = 32.0 g
= 32.0 g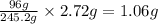 of
of 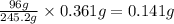 of
of 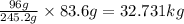 of
of  = 32731 g of
= 32731 g of 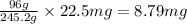
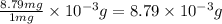 of
of 

