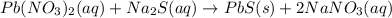The solutions of Pb(NO3)2(aq) and Na2S(aq) represented above are mixed, causing a precipitate to
To form. The Balanced equalion for the actions shown below
Pb(NO3)2 (aq) + Na2S(aq) Pbs(s) + 2 NaNO3(aq)
which of the following diagrams best represents the mixture after the reaction has proceeded as
Completely as possible?
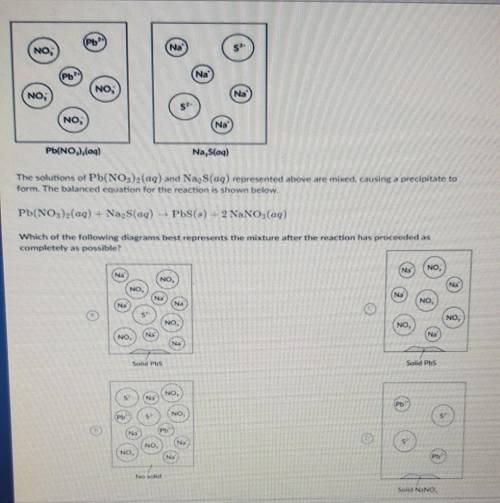

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1



You know the right answer?
The solutions of Pb(NO3)2(aq) and Na2S(aq) represented above are mixed, causing a precipitate to
To...
Questions in other subjects:

History, 11.02.2022 05:50

History, 11.02.2022 05:50


Mathematics, 11.02.2022 05:50

Mathematics, 11.02.2022 05:50


Mathematics, 11.02.2022 05:50


Chemistry, 11.02.2022 05:50