Answers: 1


Other questions on the subject: Chemistry



Chemistry, 23.06.2019 07:20, prettydoll19
Which statement explains which component is likely to be more powerful in explaining a scientific phenomenon? a) component c, because a theory is often passed on possibility and not certainty b) component d, because a hypothesis is often based on possibility not certainty c) component c, because the ability to explain several occurrences in the natural world is a characteristic of a hypothesis d) component d, because the ability to explain several occurrences in the natural world is a characteristic of a theory
Answers: 3

Chemistry, 23.06.2019 15:40, hillmarilyn70pe8sy6
Sugar is made up of clear, colorless crystals that dissolve easily in water, but the crystals and their solution do not conduct electricity. which statement describes sugar? it is made up of atoms that are held together by metallic bonds. it is made up of atoms that are held together by covalent bonds, it is made up of atoms that are held together by weak ionic bonds. it is made up of atoms that are held together by strong ionic bonds.
Answers: 1
You know the right answer?
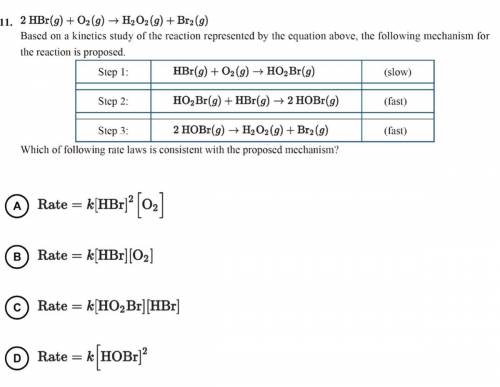
2 HBr(g)+O2(g)—>H2O2(g)+Br2(g)
Based on a kinetics study of the reaction represented by the equ...
Questions in other subjects:


English, 16.09.2019 08:30





Health, 16.09.2019 08:30

Social Studies, 16.09.2019 08:30

Spanish, 16.09.2019 08:30

Biology, 16.09.2019 08:30