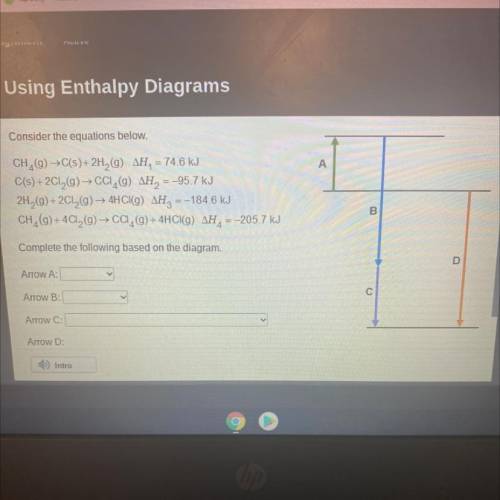
Consider the equations below.
CH,(9) →C(s)+2H2 (9) AH, = 74.6 kJ
C(s) + 2Cl2(g) → CCI (9) AH2...

Chemistry, 03.03.2021 01:10 angelashaw449
Consider the equations below.
CH,(9) →C(s)+2H2 (9) AH, = 74.6 kJ
C(s) + 2Cl2(g) → CCI (9) AH2 = -95.7 kJ
2H2(g) + 2Cl2(g) → 4HCI(9) AH2 = -184.6 kJ
CH, (g) + 4C12(g) → CCI,(9) + 4HCl(g) AH, = -205.7 kJ
Complete the following based on the diagram.
Arrow A:
Arrow B:
Arrow C:
Arrow D:

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, tiniecisneros28
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1

Chemistry, 22.06.2019 21:20, 50057543
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 23.06.2019 01:30, sheldonwaid4278
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1

Chemistry, 23.06.2019 01:30, mindofnyny
Which is an example of a highly unstable isotope that is often used in fission reactions?
Answers: 1
You know the right answer?
Questions in other subjects:



Social Studies, 13.10.2019 00:30

Mathematics, 13.10.2019 00:30



Mathematics, 13.10.2019 00:30


Mathematics, 13.10.2019 00:30

History, 13.10.2019 00:30



