
Chemistry, 02.03.2021 23:40 MalikaJones
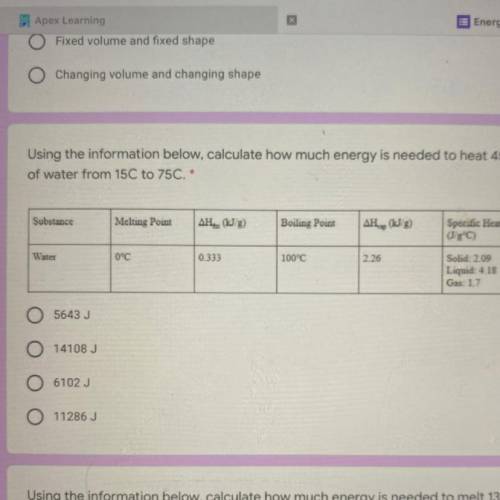
Using the information below, calculate how much energy is needed to heat 45 g
of water from 15C to 75C. *
1. 5643 J
2. 14108 J
3. 6102 J
4. 11286J

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 20:20, mgavyn1052
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1

Chemistry, 22.06.2019 00:00, chameleonsarelife
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2
You know the right answer?
Using the information below, calculate how much energy is needed to heat 45 g
of water from 15C to...
Questions in other subjects:


English, 06.05.2020 00:28

History, 06.05.2020 00:28

Social Studies, 06.05.2020 00:28



Mathematics, 06.05.2020 00:28

English, 06.05.2020 00:28




