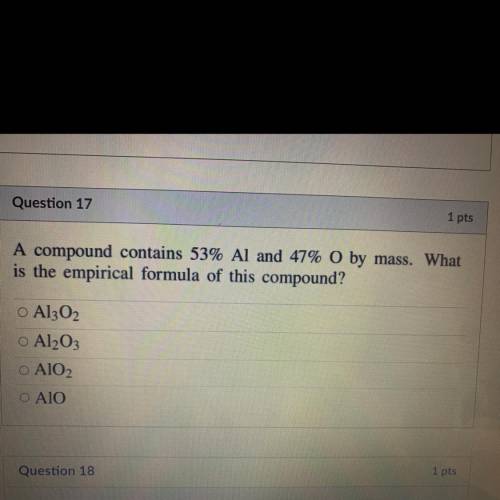
A compound contains 53% Al and 47% O by mass. What
is the empirical formula of this compound?...


Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, damienlopezram
You 4. you have been swimming in your neighbor’s pool for an hour. the relative humidity of the air is 30 percent. will you feel warm or cool when you step out of the pool? explain your answer.
Answers: 1

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 09:00, hellodarkness14
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 23.06.2019 00:20, jessicamcummins
What type of context clue you understand the meaning of quandary?
Answers: 3
You know the right answer?
Questions in other subjects:




English, 17.07.2019 09:30


Advanced Placement (AP), 17.07.2019 09:30

Mathematics, 17.07.2019 09:30


Mathematics, 17.07.2019 09:30

Mathematics, 17.07.2019 09:30