Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lukeakalucas
Alarge marble is dropped in a graduated cylinder with 35ml of water in it. the water level increases to 49ml. what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 12:00, shifaxoxoxo
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 19:20, evansh78
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 22:30, arodavoarodavo
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3
You know the right answer?
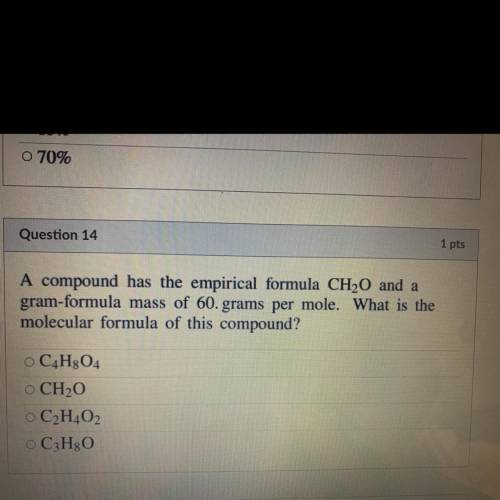
A compound has the empirical formula CH20 and a
gram-formula mass of 60. grams per mole. What is th...
Questions in other subjects:


Mathematics, 14.04.2021 17:00


Biology, 14.04.2021 17:00


English, 14.04.2021 17:00


Spanish, 14.04.2021 17:00

History, 14.04.2021 17:00

Mathematics, 14.04.2021 17:00