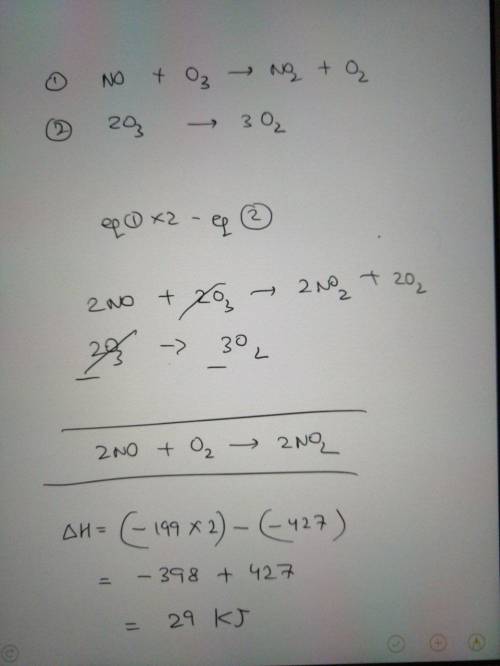Nitric oxide gas, NO(g), can be oxidized in air to
produce nitrogen dioxide gas, NO2(g):
2 NO...

Chemistry, 02.03.2021 14:40 dinosaur10
Nitric oxide gas, NO(g), can be oxidized in air to
produce nitrogen dioxide gas, NO2(g):
2 NO(g) + O2(g) → 2 NO2(g)
Determine the enthalpy change for this reaction
using any of these thermochemical equations:
02(g) →20(g)
AH = +495 kJ

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 19:20, halledoll2002
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
Questions in other subjects:


Mathematics, 30.03.2020 03:53



World Languages, 30.03.2020 03:53


English, 30.03.2020 03:53

History, 30.03.2020 03:53


Mathematics, 30.03.2020 03:53