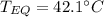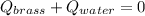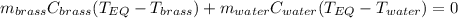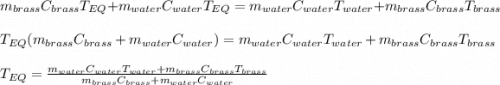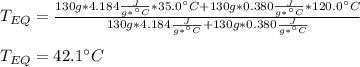Chemistry, 02.03.2021 14:00 LarryJoeseph
A 130 g sample of brass at 120.0 degrees Celsius is placed in a calorimeter cup that contains
130 g of water at 35.0 degrees Celsius. Disregard the absorption of heat by the cup and
calculate the final temperature of the brass and water. Specific heat of water = 4.18 J/gC,
specific heat of brass=0.380 J/gC. Attach your complete solution

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:30, losalobos46
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 19:50, VoidedAngel
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
A 130 g sample of brass at 120.0 degrees Celsius is placed in a calorimeter cup that contains
130 g...
Questions in other subjects:

History, 25.07.2019 21:00


English, 25.07.2019 21:10






SAT, 25.07.2019 21:10

Mathematics, 25.07.2019 21:10