Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:50, josie311251
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3

Chemistry, 23.06.2019 01:00, akluke6059
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1

Chemistry, 23.06.2019 05:00, jjoyner
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2
You know the right answer?
PLEASE HELP
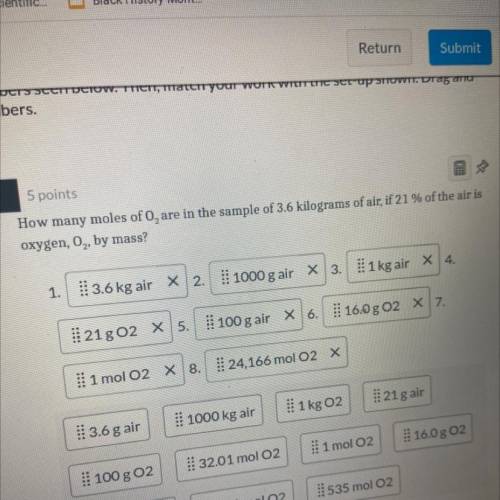
How many moles of O2 are in the sample of 3.6 kilograms of air, if 21% of the air is ox...
Questions in other subjects:

Mathematics, 14.04.2021 20:10

History, 14.04.2021 20:10

Engineering, 14.04.2021 20:10



Mathematics, 14.04.2021 20:10

Mathematics, 14.04.2021 20:10


Mathematics, 14.04.2021 20:10

Health, 14.04.2021 20:10