
Chemistry, 01.03.2021 22:00 guzmangisselle
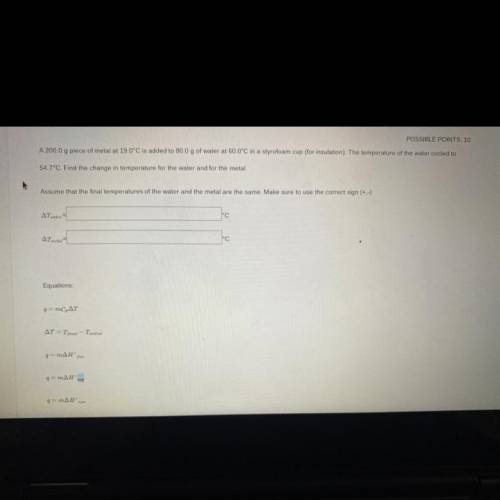
a 200g peice of metal at 19C is added to 80g of water at 60C in a styrofoam cup(for insulation). the temperature of the water is cooled at 54.7C. Find the temperature for the water and the metal. Assume that the final temperatures for the water and the metal are the same. make sure to use the correct sign

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, codeyhatch142
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1



Chemistry, 22.06.2019 21:30, starl0rd211
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2
You know the right answer?
a 200g peice of metal at 19C is added to 80g of water at 60C in a styrofoam cup(for insulation). the...
Questions in other subjects:

Mathematics, 14.08.2020 20:01

Mathematics, 14.08.2020 20:01



Mathematics, 14.08.2020 20:01



Mathematics, 14.08.2020 20:01




