Chemistry, 01.03.2021 21:50 nocomprendoplshelp
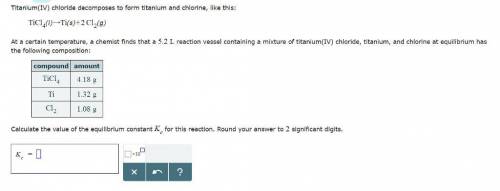
Titanium(IV) chloride decomposes to form titanium and chlorine, like this:
TiCl4(l) → Ti(s)+ 2Cl2(g)
At a certain temperature, a chemist finds that a reaction vessel containing a mixture of titanium(IV) chloride, titanium, and chlorine at equilibrium has the following composition:
Compound Amount
TiCl4 4.18g
Ti 1.32g
Cl2 1.08g
Required:
Calculate the value of the equilibrium constant for this reaction.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, lrasanaoaksandfurana
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1

Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 12:00, macylen3900
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2
You know the right answer?
Titanium(IV) chloride decomposes to form titanium and chlorine, like this:
TiCl4(l) → Ti(s)+ 2Cl2(g...
Questions in other subjects:

Spanish, 01.03.2021 05:00

Mathematics, 01.03.2021 05:00


Mathematics, 01.03.2021 05:00

History, 01.03.2021 05:00


Chemistry, 01.03.2021 05:00