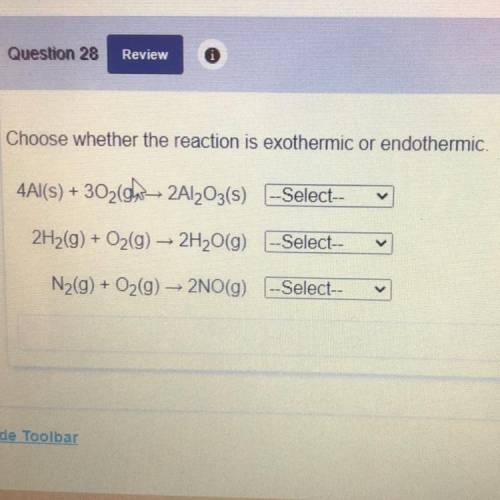
Choose whether the reaction is exothermic or endothermic.
4Al(s) + 3020w 2Al2O3(s) --Select--
...

Chemistry, 01.03.2021 20:10 ilovecatsomuchlolol
Choose whether the reaction is exothermic or endothermic.
4Al(s) + 3020w 2Al2O3(s) --Select--
V
2H2(g) + O2(g) → 2H2O(9) --Select--
N2(g) + O2(g) - 2NO(g) -Select--

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:00, wizz4865
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2


Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1
You know the right answer?
Questions in other subjects:


Physics, 03.11.2021 01:20



Geography, 03.11.2021 01:20


Biology, 03.11.2021 01:20

Mathematics, 03.11.2021 01:20

History, 03.11.2021 01:20

Social Studies, 03.11.2021 01:20



