Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, Gghbhgy4809
What pressure will be exerted by 0.675 moles of a gas at 25*c if it is in a 0.750-l container?
Answers: 1

Chemistry, 22.06.2019 14:00, njones58emailtjcedu
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 23.06.2019 03:00, rayne40
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3
You know the right answer?
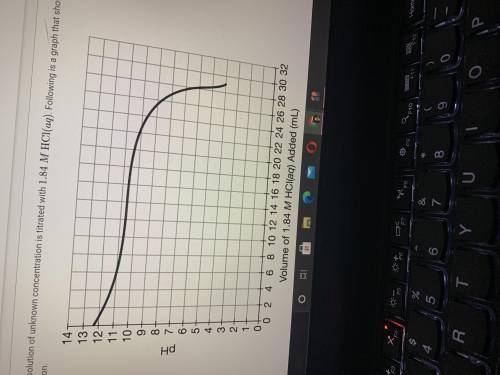
If 28.25mL of 1.84MHCl(aq) was required to reach the equivalence point, calculate the concentration...
Questions in other subjects:

Chemistry, 13.02.2021 07:40

History, 13.02.2021 07:40

Computers and Technology, 13.02.2021 07:40


Medicine, 13.02.2021 07:40

Mathematics, 13.02.2021 07:40

Mathematics, 13.02.2021 07:40

Mathematics, 13.02.2021 07:40