
Chemistry, 01.03.2021 08:00 gottapass62
How many grams of water will be produced when 32.0 g of oxygen are allowed to react with hydrogen? _H²+_O² ➡ _H²O
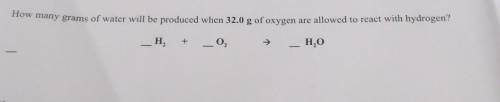

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:50, aletadaboss
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 11:00, 21villalobosjabez
Which type of fossil does this image depict?
Answers: 1

Chemistry, 22.06.2019 11:30, samantha9430
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

You know the right answer?
How many grams of water will be produced when 32.0 g of oxygen are allowed to react with hydrogen?...
Questions in other subjects:

Mathematics, 03.09.2021 22:00

Mathematics, 03.09.2021 22:00







Spanish, 03.09.2021 22:00

Mathematics, 03.09.2021 22:00



