Chemistry, 28.02.2021 14:50 nataliemoore1974
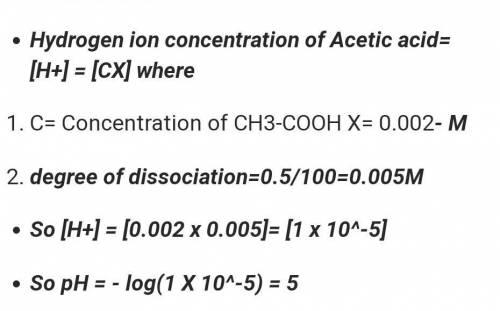
Calculate the pH of a 0.002 M acetic acid solution if it is 2.3% ionised at this dilution. Ka = 1.8 x 10-5.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, david838843
Iwll give extra points to who gets this for ! what type of reaction is this? ?
Answers: 2


Chemistry, 22.06.2019 23:00, 1315055427
Which subshell is represented by the actinides family?
Answers: 1
You know the right answer?
Calculate the pH of a 0.002 M acetic acid solution if it is 2.3% ionised at this dilution. Ka = 1.8...
Questions in other subjects:

Mathematics, 30.11.2020 18:50

Mathematics, 30.11.2020 18:50






Mathematics, 30.11.2020 18:50

Mathematics, 30.11.2020 18:50