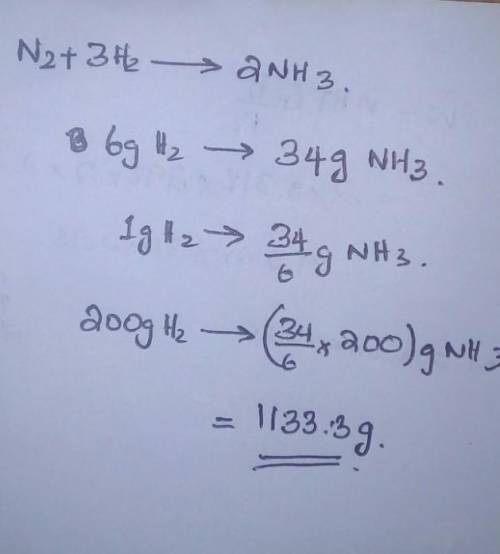Chemistry, 28.02.2021 14:00 tednequamoore6761
H2 reacts with N2 to produce NH3 according to the equation N2 + 3 H2 2NH3 . Determine how much NH3 would be produced if 200 g H2 react ?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, mykalwashington
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 22.06.2019 03:00, bchagnard2122
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 05:30, livigrace9004
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2
You know the right answer?
H2 reacts with N2 to produce NH3 according to the equation N2 + 3 H2 2NH3 . Determine how much NH3 w...
Questions in other subjects:

Mathematics, 28.05.2020 00:00

History, 28.05.2020 00:00



Physics, 28.05.2020 00:00

French, 28.05.2020 00:00

Mathematics, 28.05.2020 00:00

Mathematics, 28.05.2020 00:00

Mathematics, 28.05.2020 00:00