
Chemistry, 28.02.2021 06:40 twistedhyperboles
2c+02=2CO2. The moles of co2 produced when 0.25 moles of O2 react is?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, huangjianhe135
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 05:30, madisonrosamond99
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2


Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1
You know the right answer?
2c+02=2CO2. The moles of co2 produced when 0.25 moles of O2 react is?...
Questions in other subjects:

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

English, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

Biology, 16.09.2020 02:01

Mathematics, 16.09.2020 02:01

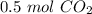
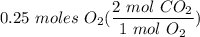 [DA] Multiply/Divide [Cancel out units]:
[DA] Multiply/Divide [Cancel out units]: 

