
Chemistry, 27.02.2021 14:10 kris22elizondop9v1bb
Calculate the PH of a 0.002M acetic acid solution if it is 2.3% ionised at this dilution ka=1.8×10⁸
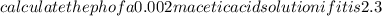

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 22:00, braydentillery1221
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1


Chemistry, 22.06.2019 11:30, claudr03
If we compare and contrast electromagnetic waves with sound waves, all but one statement is true. that is a) sound waves require a medium to travel while electromagnetic waves do not. b) electromagnetic waves can travel through the vacuum of space while sound waves cannot. c) electromagnetic waves must have a medium in which to travel, but sound waves can travel anywhere. eliminate d) sound waves must bounce off of matter in order to travel while electromagnetic waves do not require matter to be present.
Answers: 3
You know the right answer?
Calculate the PH of a 0.002M acetic acid solution if it is 2.3% ionised at this dilution ka=1.8×10⁸...
Questions in other subjects:

Chemistry, 18.10.2021 08:40

Mathematics, 18.10.2021 08:40

Mathematics, 18.10.2021 08:40


English, 18.10.2021 08:40

Physics, 18.10.2021 08:40

Biology, 18.10.2021 08:40


Mathematics, 18.10.2021 08:40



