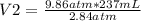Chemistry, 27.02.2021 14:00 fansofboys
A sample of nitrogen gas has a volume of 237mL at 9.86atm. What is the new volume of the gas when the pressure is changed to 2.84atm?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1


Chemistry, 23.06.2019 05:40, Queenquestion9130
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1
You know the right answer?
A sample of nitrogen gas has a volume of 237mL at 9.86atm. What is the new
volume of the gas when t...
Questions in other subjects:

Arts, 07.09.2020 01:01


Mathematics, 07.09.2020 01:01


Mathematics, 07.09.2020 01:01

Spanish, 07.09.2020 01:01

Mathematics, 07.09.2020 01:01

SAT, 07.09.2020 01:01


Mathematics, 07.09.2020 01:01