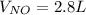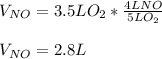Chemistry, 27.02.2021 08:00 georgesarkes12
4NH3(g) + 5O2(g) -> 4NO(g) +6H20(g) If 3.5 L of oxygen gas at STP react with an excess amount of ammonia, how many liters of nitrogen monoxide will be produced

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, shanicar33500
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1


Chemistry, 23.06.2019 02:00, xoxoadara13ox07ck
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
4NH3(g) + 5O2(g) -> 4NO(g) +6H20(g) If 3.5 L of oxygen gas at STP react with an excess amount of...
Questions in other subjects:

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Social Studies, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Social Studies, 17.09.2020 14:01

English, 17.09.2020 14:01

Health, 17.09.2020 14:01

History, 17.09.2020 14:01