Type of Solutions and Factors that Affect
them
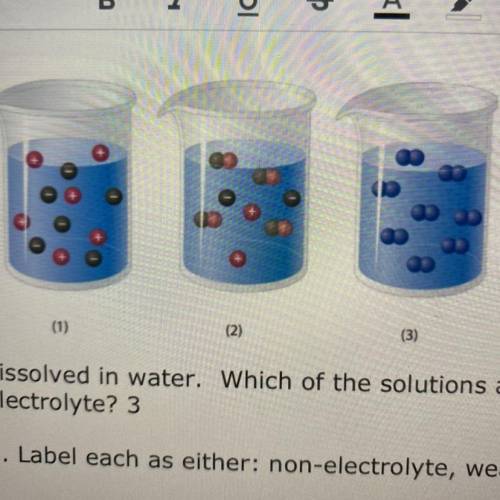
1. The diagram above represents solute partic...

Type of Solutions and Factors that Affect
them
1. The diagram above represents solute particles
dissolved in water. Which of the solutions above
(1,2,3) would be considered an electrolyte?
2. Label each as either: non-electrolyte, weak
electrolyte, or strong electrolyte
(1)
(2)
(3)
2. A chemist dissolves solid potassium chloride in a given amount of water until no more will
dissolve at that temperature. How could that solution be described?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, gatorr2010
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3



Chemistry, 22.06.2019 09:10, cheesedoodle
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 20.11.2019 07:31


Chemistry, 20.11.2019 07:31

Health, 20.11.2019 07:31


Health, 20.11.2019 07:31






