
Chemistry, 26.02.2021 17:50 terrancebest
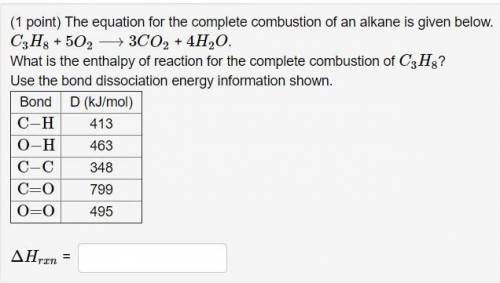
(1 point) The equation for the complete combustion of an alkane is given below.
C3H8 + 5O2 ⟶ 3CO2 + 4H2O.
What is the enthalpy of reaction for the complete combustion of C3H8?
Use the bond dissociation energy information shown.
Bond D (kJ/mol)
C−H 413
O−H 463
C−C 348
C=O 799
O=O 495
ΔHrxn =

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, kangasc6124
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2

Chemistry, 22.06.2019 15:00, kandi2565
Large helium-filled balloons are used to lift scientific equipment to high altitudes. what is the pressure inside such a balloon if it starts out at sea level with a temperature of 10.0ºc and rises to an altitude where its volume is twenty times the original volume and its temperature is – 50.0ºc ?
Answers: 2

Chemistry, 22.06.2019 15:20, mydoggy152
Fossil fuels are organic compounds that are made from
Answers: 1
You know the right answer?
(1 point) The equation for the complete combustion of an alkane is given below.
C3H8 + 5O2 ⟶ 3CO2 +...
Questions in other subjects:

Health, 28.09.2019 03:30


Mathematics, 28.09.2019 03:30

Mathematics, 28.09.2019 03:30


Mathematics, 28.09.2019 03:30

Mathematics, 28.09.2019 03:30



English, 28.09.2019 03:30



