
Chemistry, 26.02.2021 17:40 aurelio1121
If you respond to this with a bs answer just to get the points your just gonna get reported and lose them so don't waste your time. THIS IS DUE BY 3:00pm TODAY (2/26/2021). Thanks in advance!
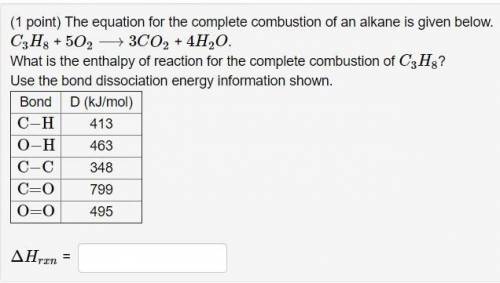

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, penny3109
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1


You know the right answer?
If you respond to this with a bs answer just to get the points your just gonna get reported and lose...
Questions in other subjects:





Biology, 09.02.2021 18:00


Mathematics, 09.02.2021 18:00

Mathematics, 09.02.2021 18:00

English, 09.02.2021 18:00



